
The faster the reaction and the more energy released during that reaction the greater the reactivity of that metal element. The rate of reaction and the energy released during a reaction is directly related to the metal's reactivity. Metal elements which can lose electrons more easily than Hydrogen is able to lose its electron will react with water and with acids.
Reactivity series of metals series#
The Reactivity Series can be found experimentally by observing the reactions between metals and either water or acid. The more easily a metal can lose electrons to become a metal ion the more reactive that metal is.
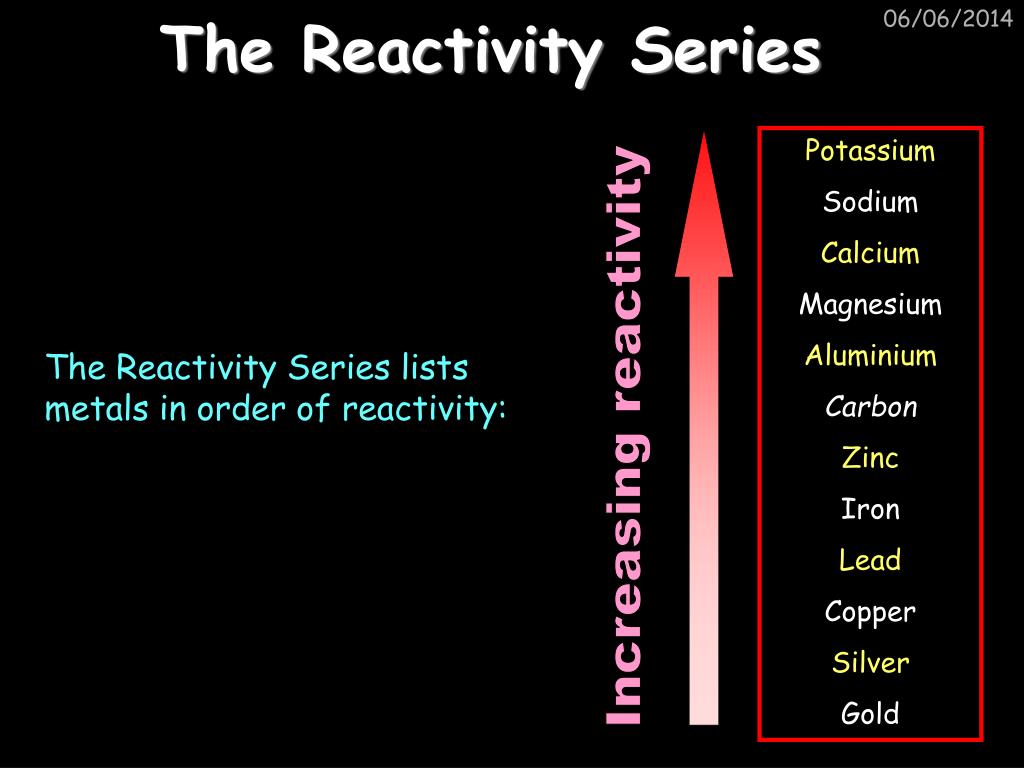
When metals are placed in order of their reactivity it is called the Reactivity Series. The Reactivity Series is a list of metal elements in order of how easily they can lose electrons to form positive ions.Ībout The Reactivity Series The reactivity of a metal is determined by how easily it can lose electrons to form positive ions. Elements above Carbon are found in minerals but they cannot be extracted with Carbon because Carbon is less reactive than those metals so we use electrolysis to extract them. Elements above Hydrogen but below Carbon are found in minerals, which are metal compounds, so they need to be extracted by using Carbon to displace the metal from the compound. Elements below Hydrogen are found Native which means the metal element can be found not as part of a compound.

Carbon and Hydrogen are important in the Reactivity Series as they can indicate how a metal can be extracted from a mineral. Elements higher on the reactivity series will displace those lower down on the reactivity series. The Reactivity Series is a list of elements in order of their reactivity.Ībout The Reactivity Series The Reactivity Series is used to predict the outcome of Displacement Reactions. The relative reactivity of metals can be determined using a number of. The symbols and atomic numbers of some elements in the reactivity series. An activity series is a list of substances in order from most reactive to least reactive.


 0 kommentar(er)
0 kommentar(er)
